Introducing Kronus a Clinical Trial
Management (CTMS) Platform
A comprehensive web-based solution for efficient clinical trial management—featuring simplified data capturing, automated workflows and real-time insights - all whilst maintaining regulatory compliance and adherence.
Companies that use Kronus






































Kronus Features
What streamlines your work?
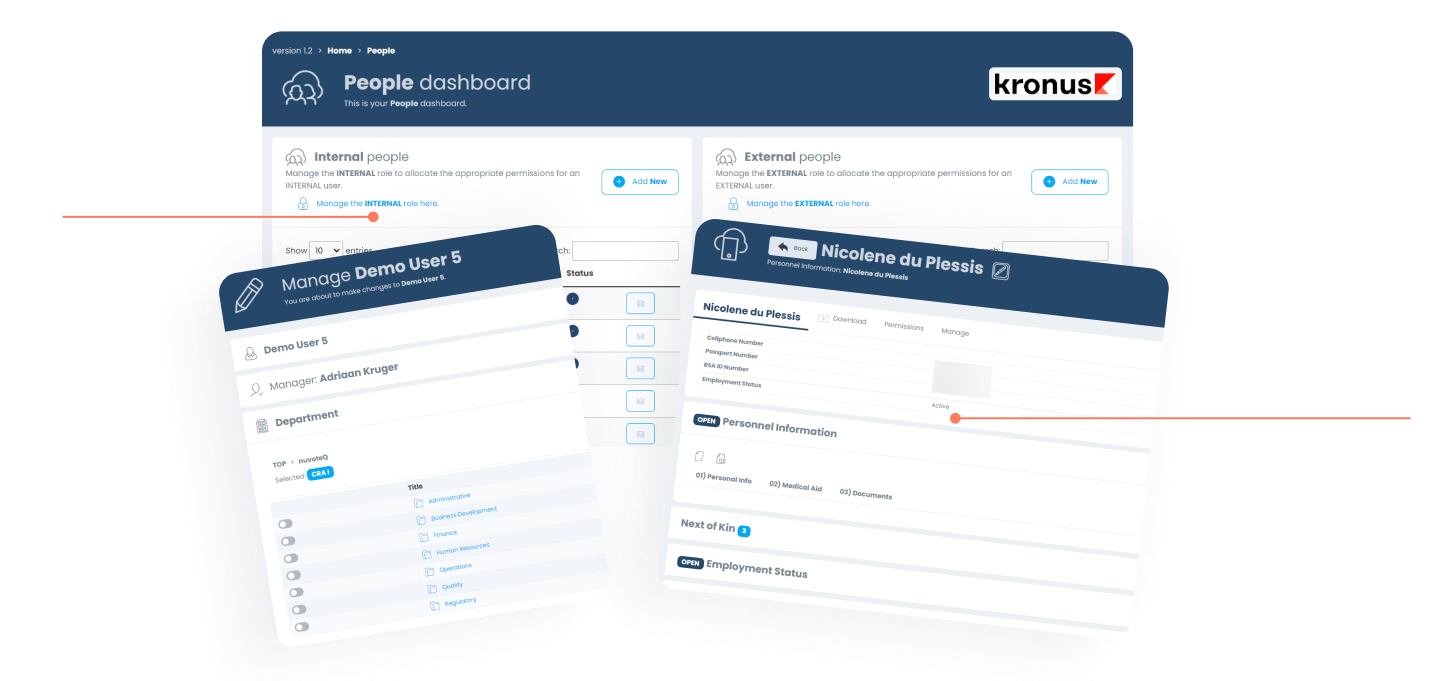
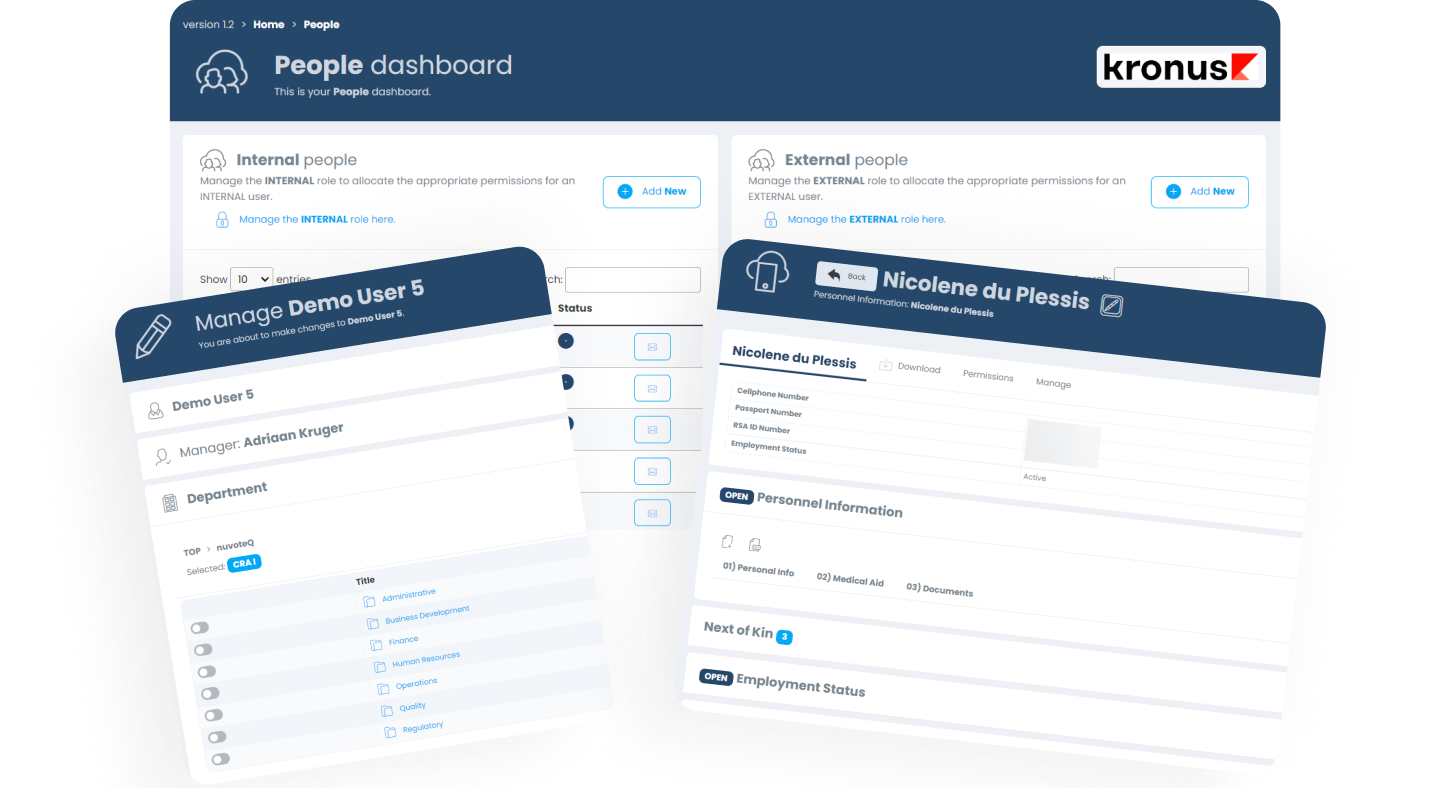
People Module
The PEOPLE module of the KRONUS platform includes the core functionality one would find in most CTMS solutions today. The module is completely integrated with the rest of platform and forms part of the foundation which makes the platform work.
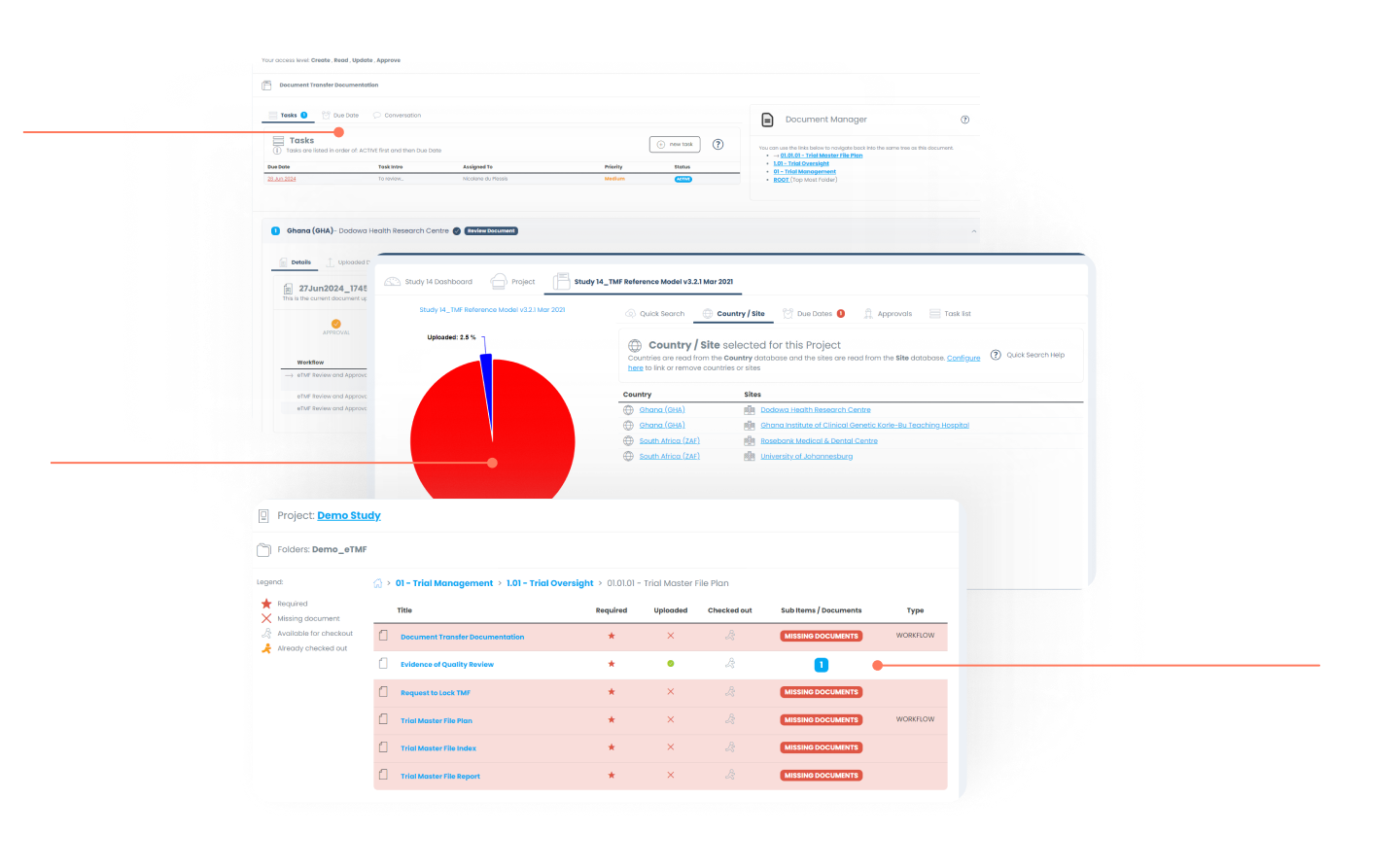
Document Management
FDA validated structure to maintain (multiple different structured) electronic Trial Master File & Investigator Site File. All access managed with role- based access and tracked with detailed audit trail.
Learning Management
Our LEARNING MANAGEMENT module is mainly focused on the administration, documentation, tracking, reporting, and delivery of SOPs, Policies, Manuals, Templates, educational courses, training programs, or learning and development programs via electronic acknowledgements or assessment. Compulsory or elective
Master Database Management
Our DATABASE module is an integral part of our KRONUS platform. The Database module forms the foundation of our bigger CTMS platform and allows automated dependency management between items. Centralized interface where structure of all lookup databases can be defined and managed (i.e. Investigators, Sites, Sponsors, Regulators, IRB, Vendors, Quality etc.).
CV Builder
The system is designed to allow for a predefined CV template to be configured according to the client’s existing template and for the CVs to be completed, reviewed and approved in an electronic format.
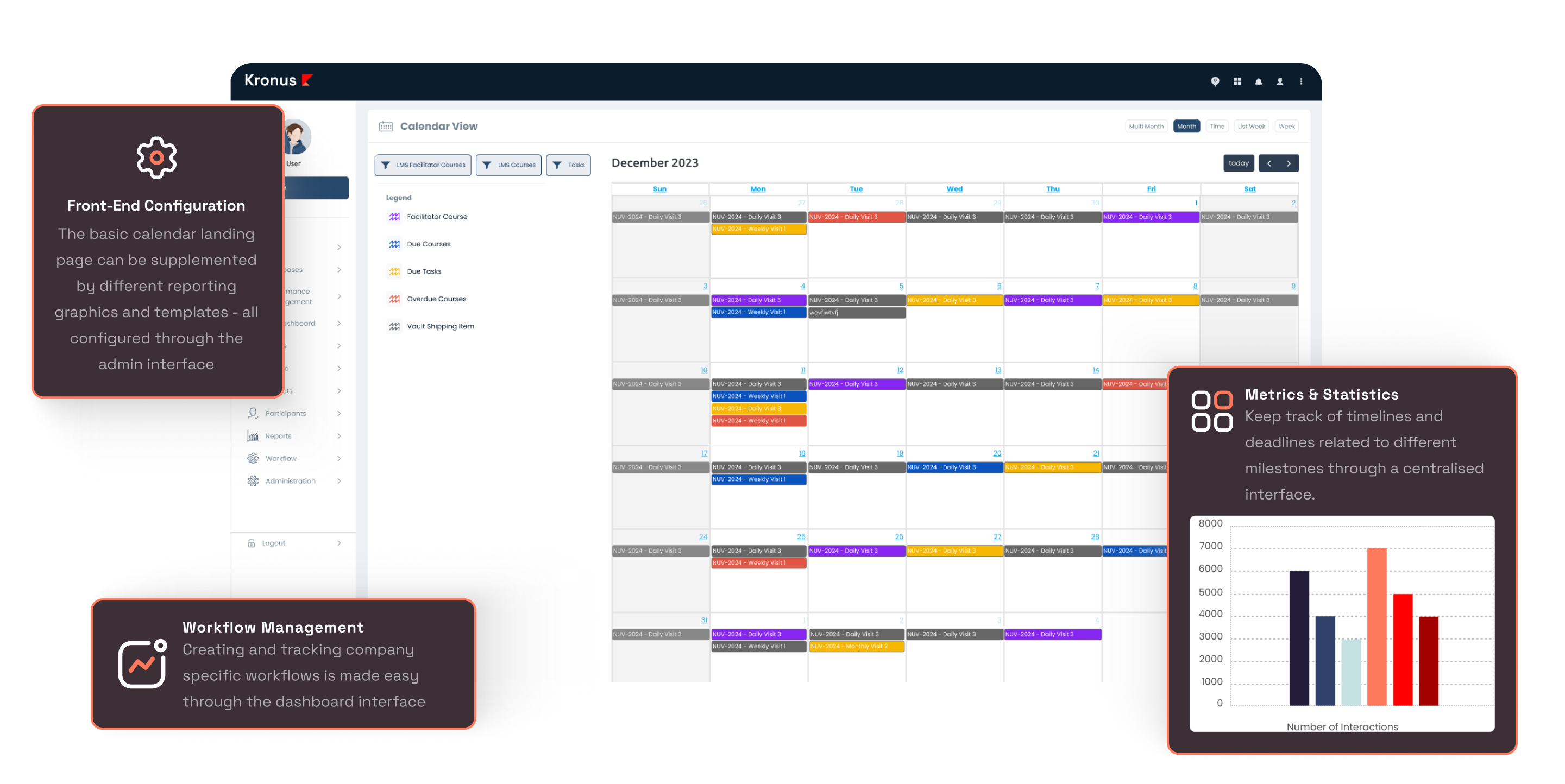
Visit Scheduler
Our SCHEDULER module is an integral part of study management. The Scheduler module allows for unique visit scheduler events to be configured as per the study protocol. With a single calendar view showing the automated schedule of events across multiple studies, it enables the study teams to plan and manage studies more efficiently and be compliant with milestone tracking.
Featured Features
People Module
The PEOPLE module of the KRONUS platform includes the core functionality one would find in most CTMS solutions today. The module is completely integrated with the rest of platform and forms part of the foundation which makes the platform work.
Office 365 Integration
The platform can seamlessly integrate with your Azure Active Director allowing users to use their work email addresses and passwords to access the portal
Organogram
Creating your company structure is very easy and allows for reporting structures and departments to be integrated with the eLearning and Performance Management module(s).
Featured Features
Document Management
FDA validated structure to maintain (multiple different structured) electronic Trial Master File & Investigator Site File. All access managed with role- based access and tracked with detailed audit trail.
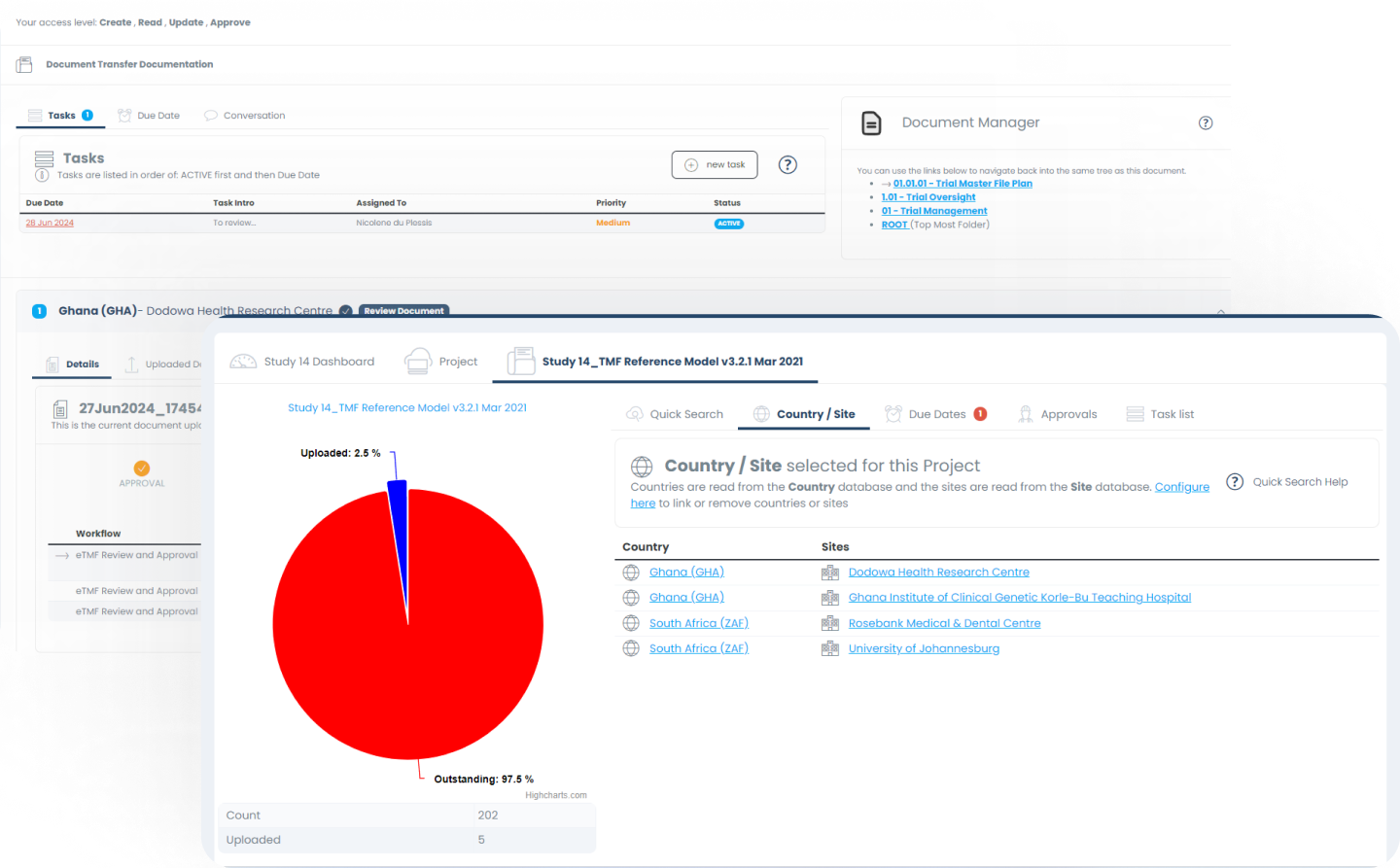
Task Management / Assignment
Using the repository of registered users on the platform one can easily assign specific tasks and deadline to users throughout the system
Dashboard Tracking
Understanding progress the progress of different tasks / milestones within the Project management is made easy through our dashboarding elements.
Electronic Trial Master File (eTMF)
The portal allows the client to import pre-defined eTMF templates or customize the structure based on their needs - all managed through user friendly icons and warning messages.
Featured Features
Learning Management
Our LEARNING MANAGEMENT module is mainly focused on the administration, documentation, tracking, reporting, and delivery of SOPs, Policies, Manuals, Templates, educational courses, training programs, or learning and development programs via electronic acknowledgements or assessment. Compulsory or elective


Tracking of SOPs & other Training
Managing the versions and access control to different training courses is made simple using the admin portal of the eLearning Module.
eLearning Dashboard
Keeping track of deadlines and outstanding courses is easy via the dashboard interface.
HTML5 / SCORM Content
The portal allows for external content creation Tools to be used to create interactive training content and be uploaded on the portal.
Featured Features
Master Data Management
Our DATABASE module is an integral part of our KRONUS platform. The Database module forms the foundation of our bigger CTMS platform and allows automated dependency management between items. Centralized interface where structure of all lookup databases can be defined and managed (i.e. Investigators, Sites, Sponsors, Regulators, IRB, Vendors, Quality etc.).
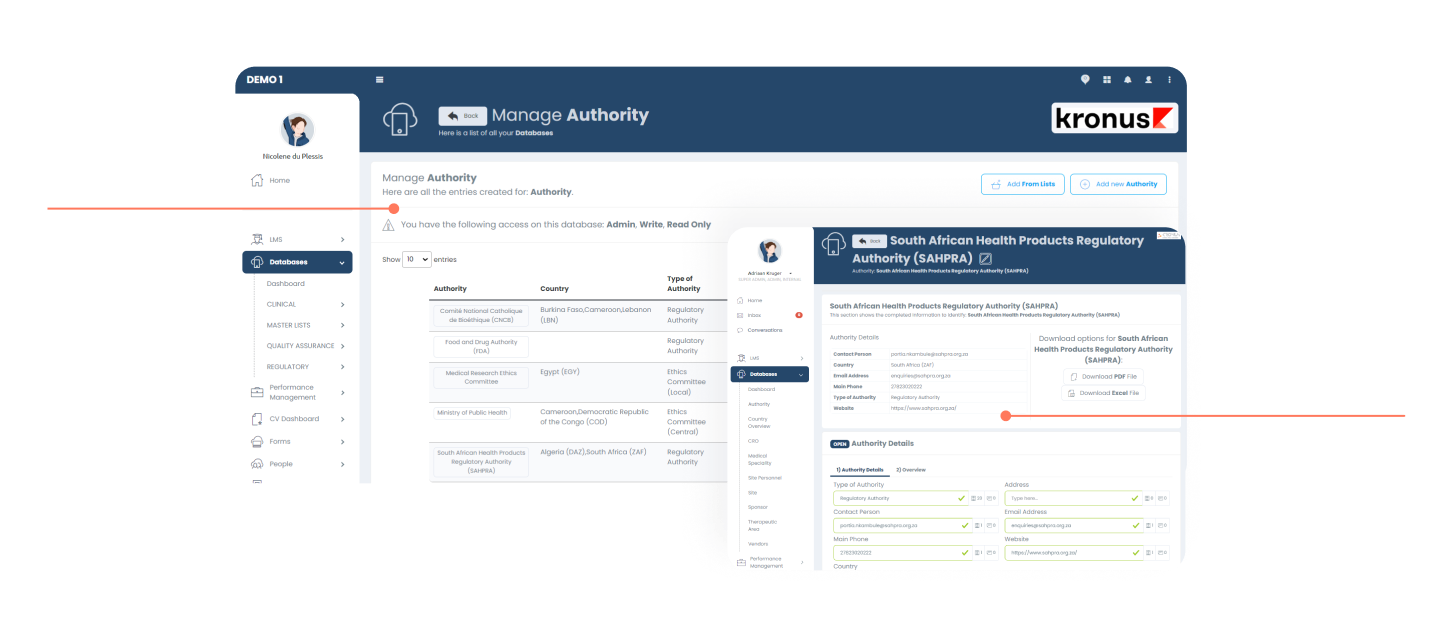
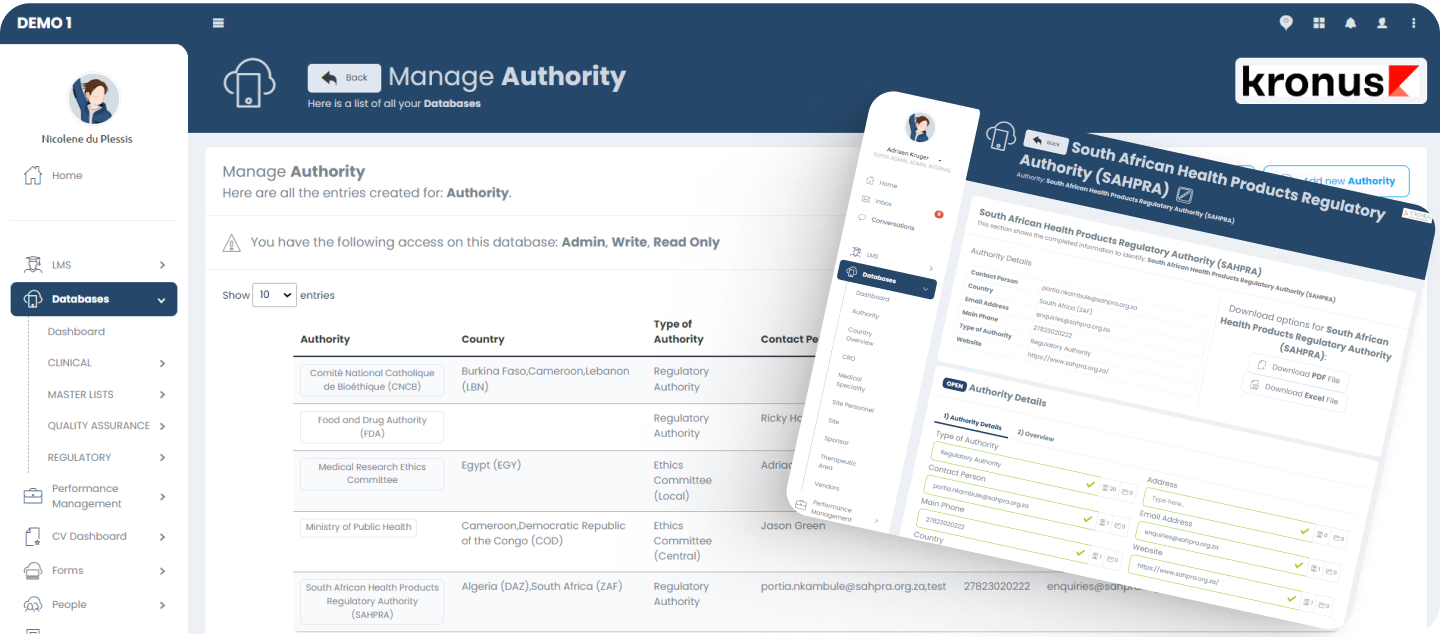
Master Data Management (MDM)
The MDM module allows customers to manage online repositories of their critical databases (i.e. Vendors, Sponsors, NRAs, etc) to be referenced in other parts of the platform.
Form Builder Tool
The flexible, yet powerful, form builder function allows for any type of form template to be configured through the front-end within minutes.
Featured Features
CV Builder
The system is designed to allow for a predefined CV template to be configured according to the client’s existing template and for the CVs to be completed, reviewed and approved in an electronic format.
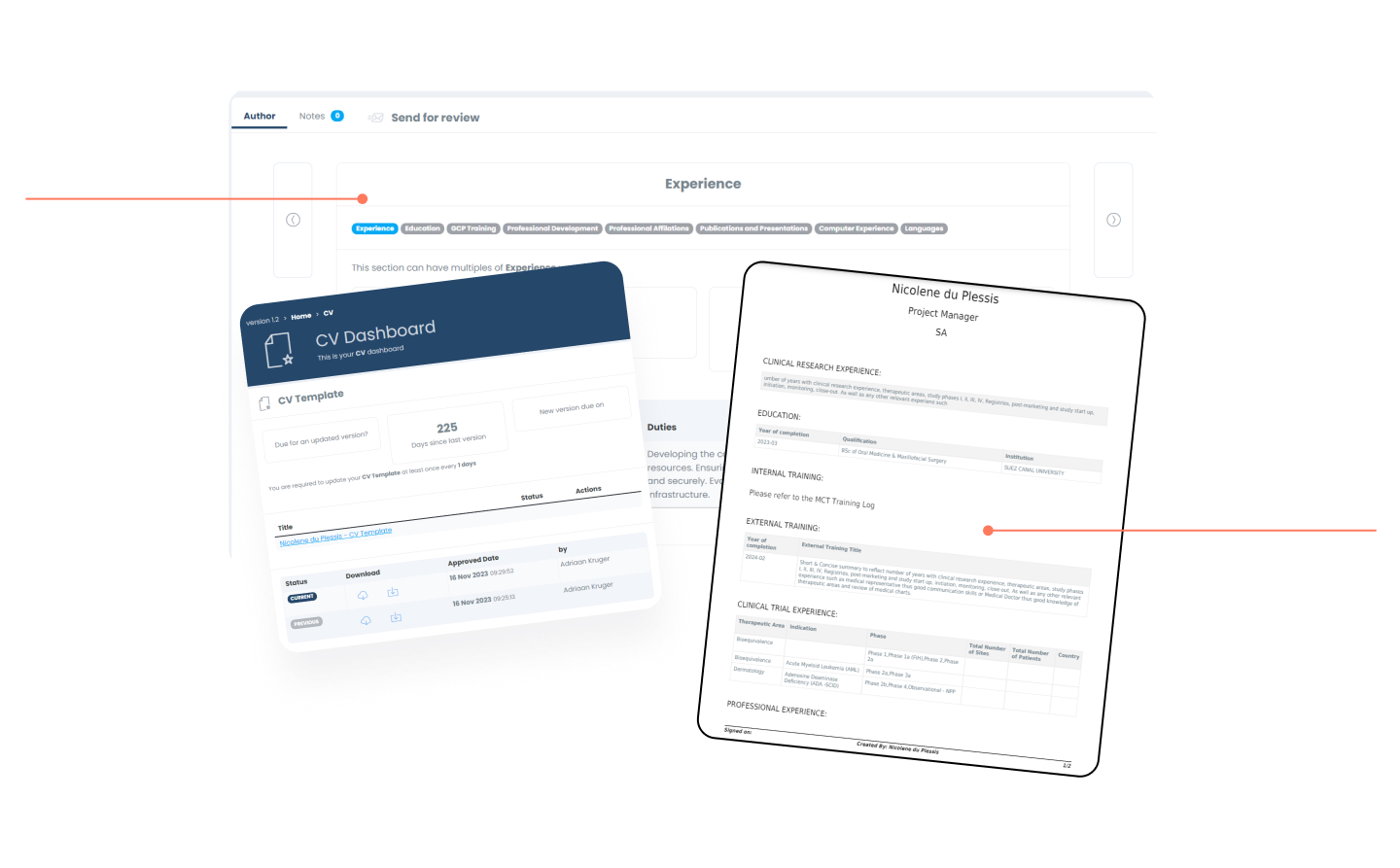
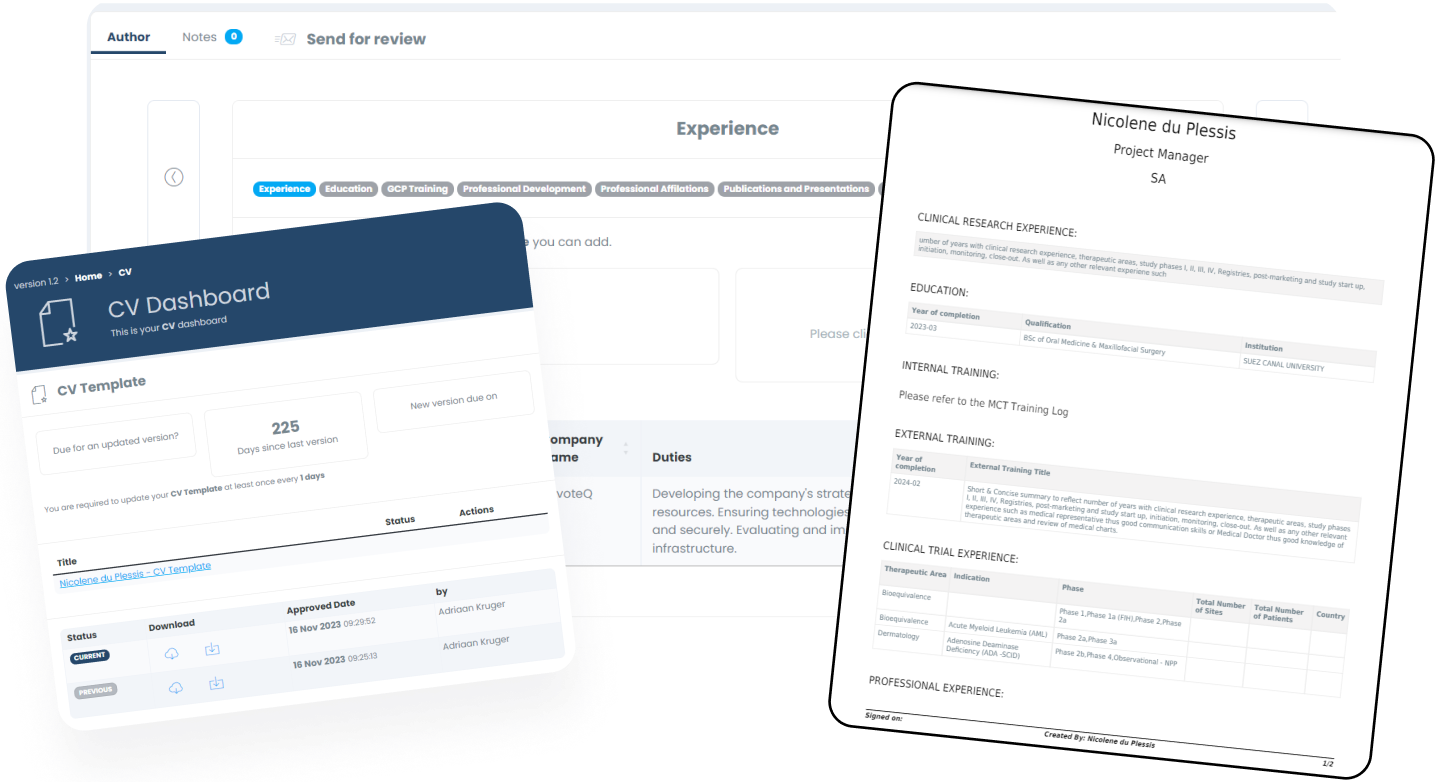
CV Builder
The interactive interface allows for users / employees to build their comprehensive CV out within minutes. The module follows a familiar flow (i.e. LinkedIn) to help make it intuitive.
CV Export Templates
Different companies / Regulators / Sponsors require different templates when it comes to CVs - that is why we designed and built the CV export tool, which allows you to configure multiple templates for all staff members.
Featured Features
Visit Scheduler
Our SCHEDULER module is an integral part of study management. The Scheduler module allows for unique visit scheduler events to be configured as per the study protocol. With a single calendar view showing the automated schedule of events across multiple studies, it enables the study teams to plan and manage studies more efficiently and be compliant with milestone tracking.
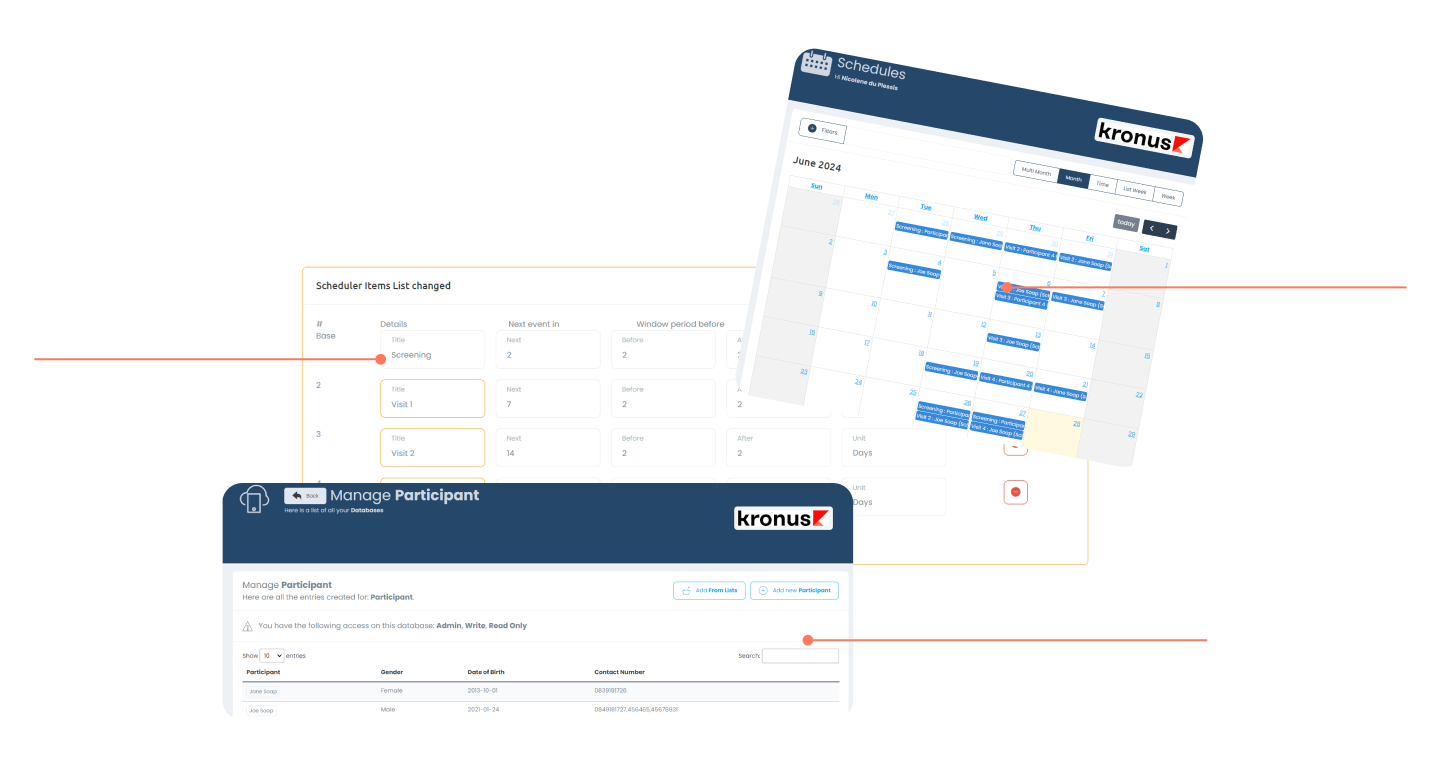
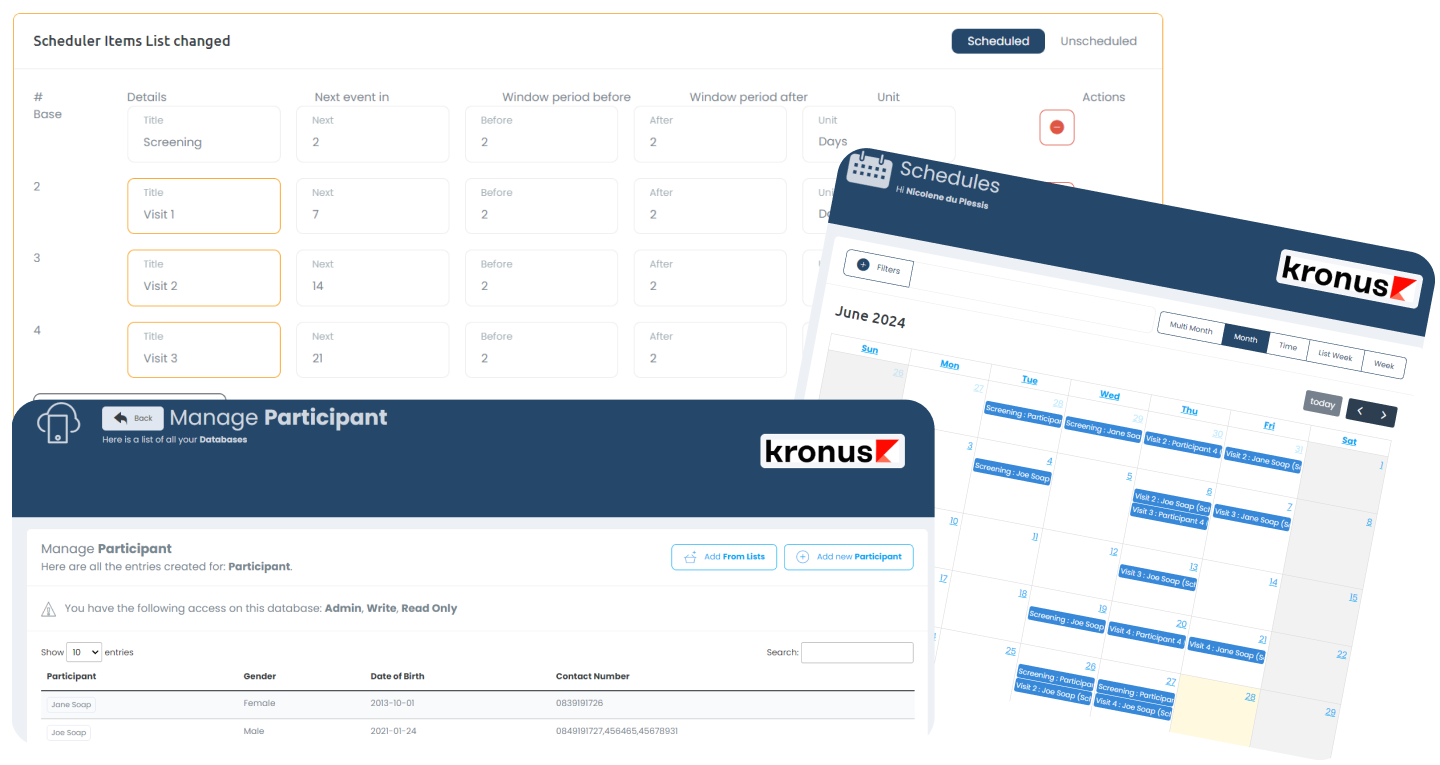
Clinic / Study Calendar
Managing the staff and IP when it comes to studies is made easy using the interactive calendar interface, which is directly linked with the Schedule of Events of the study / participant.
Visit Schedule Builder
This tool makes it easy to configure the schedule of events (including window periods) as per the protocol, and is directly linked with the Calendar interface.
Keywords