Introducing Nukleus. Ready to streamline
your clinical data collection?
Nukleus is a hybrid, secure web-based platform designed to transform clinical data collection. Whether functioning as an eSource, EDC (Electronic Data Capture), or PSP (Patient Support Program) platform, Nukleus eliminates the need for data transcription, enhancing efficiency and accuracy
Nukleus Features
What streamlines your work?
Visit Scheduler Wizard
The visit scheduler component within Nukleus takes the schedule of events (as defined within the protocol) into account and calculates the different visit dates per participant. The window periods are defined upfront and are automatically linked to edit check validators which will warn the investigator if a visit is overdue (out of window) or missed,
Integrated Medical Coding
Integrates coding libraries like MedDRA and WHO-DD ICD-10 for accurate coding of medication, medical history, and adverse event terms.
Integrated Interactive Web Response Services
Includes importing randomisation schedules, applying stratification criteria, IP allocation, replacement, and real-time randomization number generation.
Electronic Patient Reported Outcomes
Our platform allows for this to be done in the form of web-based surveys, two-way text
message communication or USSD (Unstructured Service Supplementary Data)
• This tool
assist in ensuring that relevant information is systematically contemporaneously
captured, stored and made available for assessment by trained professionals, thereby
assisting in the overall quality of the data.
Local Lab Module
Our Local Lab Module can be used to define lab reference ranges across different sites. Each site is linked to a specific laboratory which in turn is linked to a set of reference ranges. The reference ranges can be configured for different criteria (i.e., age, sex, severity of disease etc.). These reference ranges are automatically linked to our edit-check wizard allowing for out-of-range variables to be flagged as and when the lab data is entered or imported.
Low-code / No-code (Front End Configurability)
Customers can easily configure solutions without coding, including role-based access, edit checks, logic, calculations, workflows, and notifications, potentially reducing costs and time by up to 80%.
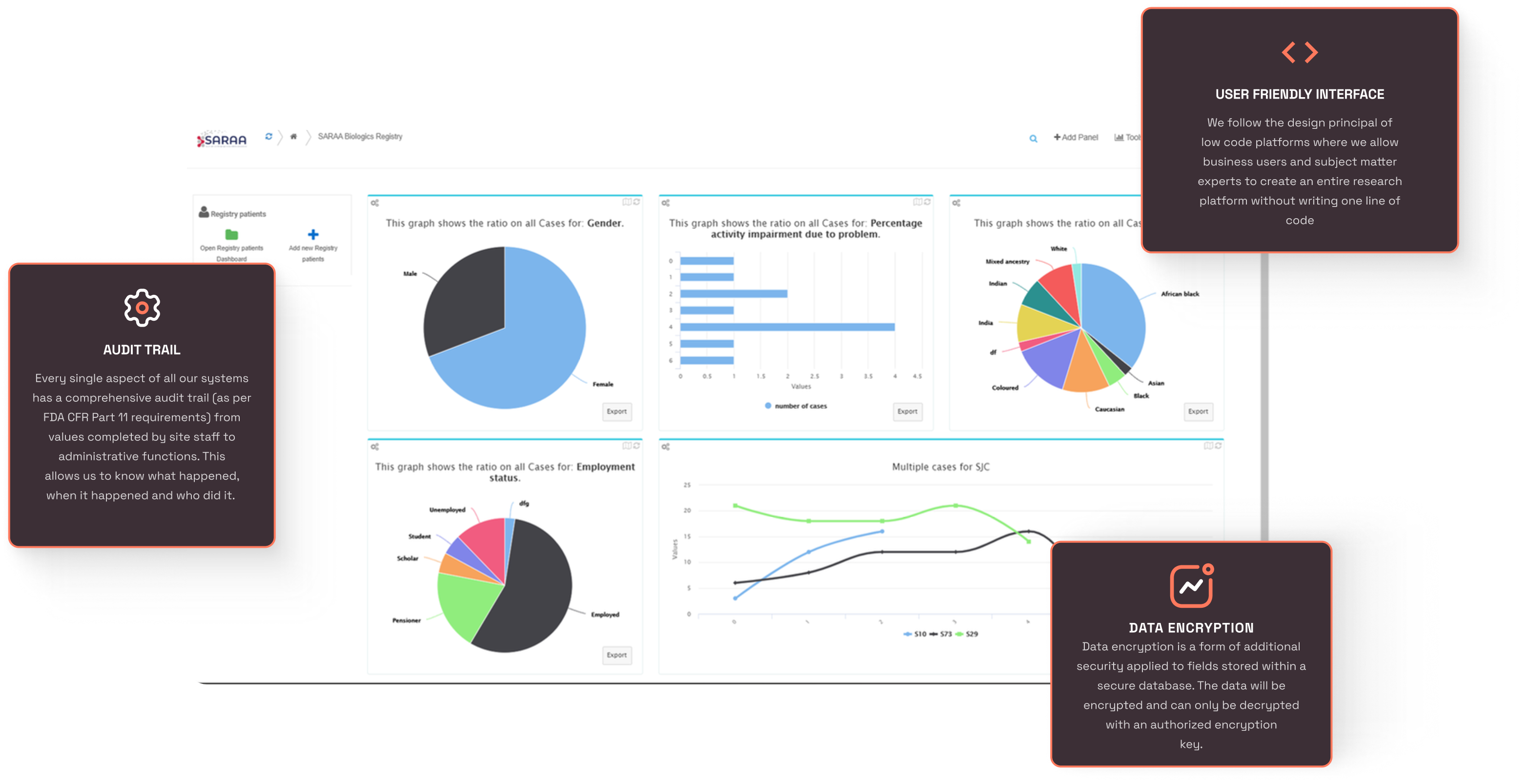
Customizable Dashboards
- Dashboard widgets are made available to all users in the system and our customized birth profile. The user can configure his or her own dashboard widget and have it personalized for the specific job description.
- The widget types include bar charts, pie charts, line charts, query rates, weather widgets and more


Rapid configuration
Our platform enables rapid configuration through a low-code design principle, allowing business users and subject matter experts to create an entire research platform without writing a single line of code. This is achieved via intuitive GUI creation tools that manage all edit checks and derived calculations directly from the front-end, pre-configured libraries of questions and procedures (pre-coded with SDTM naming standards) that can be quickly assembled within minutes, and additional front-end tools that simplify the creation of study-specific data points and procedures using a single resource with minimal effort.
Local Lab Module
Our Local Lab Module can be used to define lab reference ranges across different sites. Each site is linked to a specific laboratory which in turn is linked to a set of reference ranges. The reference ranges can be configured for different criteria (i.e., age, sex, severity of disease etc.).


Integrated Coding Libraries
Our Nukleus EDC platform supports various coding libraries, such as MedDRA, WHO DD (WIP), to accurately code concomitant medications, medical histories, and adverse event terms. These libraries are fully integrated and can be included in any solution if the sponsor company, CRO, or research facility holds the necessary licenses. Additionally, the coding is configurable, allowing users to choose the verbatim term, lower-level term, or preferred term as their primary point for data capture.
ePRO (Electronic Patient Reported Outcomes)
Our platform enables patients to complete questionnaires via web surveys, two-way text messages, or USSD (Unstructured Supplementary Service Data), with data systematically captured, stored, and made available for professional assessment to enhance quality.


Report Builder
We approached reporting in the same way we approached rapid configuration of studies. Our comprehensive reporting tools allow all stakeholders within the clinical research life cycle (Sponsors, Project Managers, Clinical Research Associates, Data Managers, Safety Physicians, etc) to build and export reports based on their interest.
Map
Compliance & Server Locations
Although we abide by the strict rules set out by industry specific regulatory authorities on developing applications storing sensitive information (i.e. FDA CFR-11, EMA Annexure 11, ICH GCP, etc), we are forward thinking in our processes and venture to produce market relevant and user-friendly systems.

Keywords