Introducing SafetyBase a web-based
pharmacovigilance platform
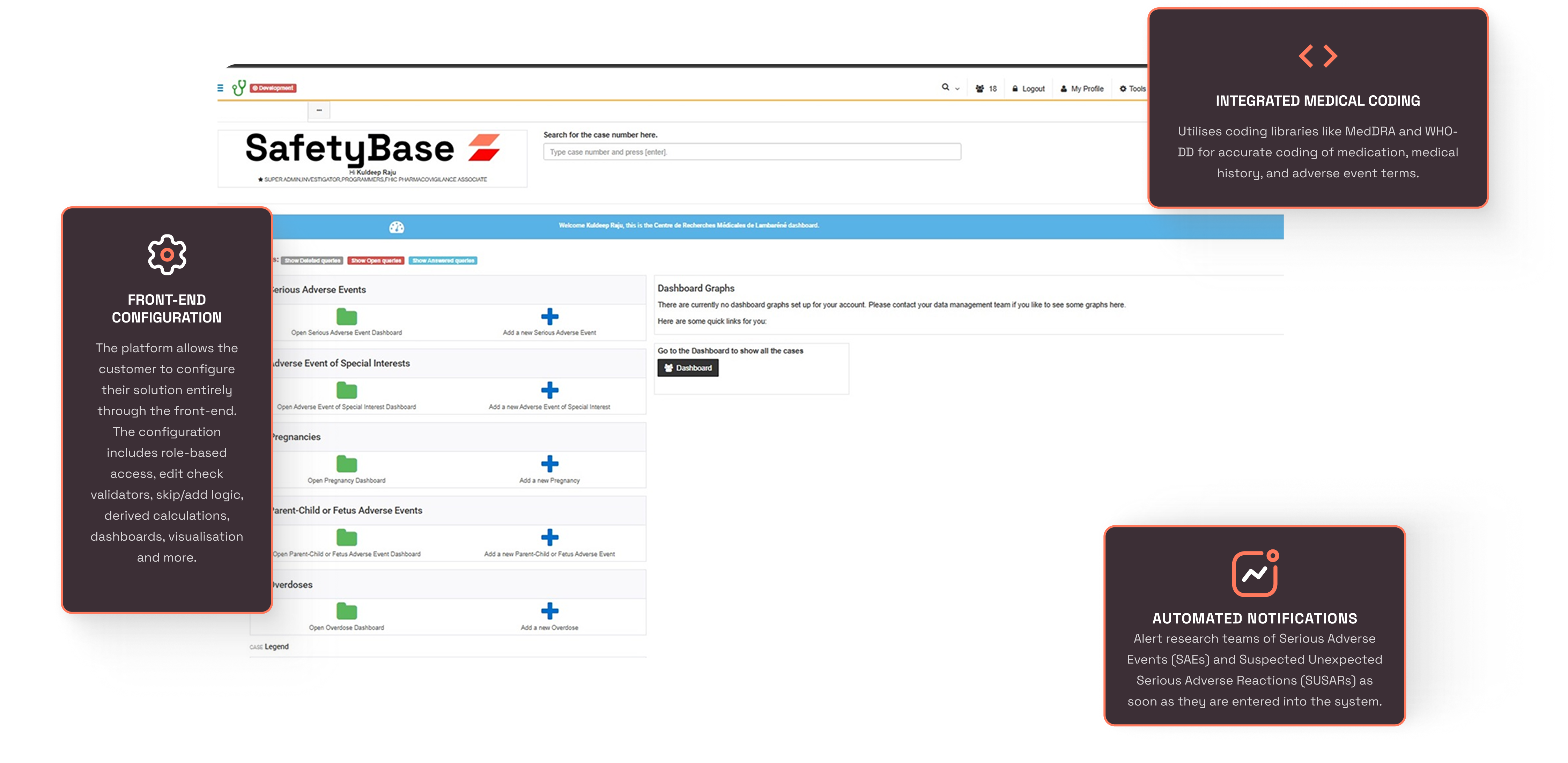
Safetybase is an advanced pharmacovigilance platform that can be accessed through modern web browsers on any device. It simplifies the collection of safety data, supporting and accelerating the development of drugs and medical devices. The platform is specifically designed to streamline the gathering of pharmacovigilance data, both during clinical trials and in post-market settings
Key Aspects
Key Kronus Features
SafetyBase Features
What streamlines your work?
Real-Time Pharmacovigilance and Quality Reporting
Streamline the collection and submission of adverse event, product complaint and medical information request data in real time. Our system ensures that safety and quality reports are captured and transmitted efficiently, enabling quicker responses to emerging issues.
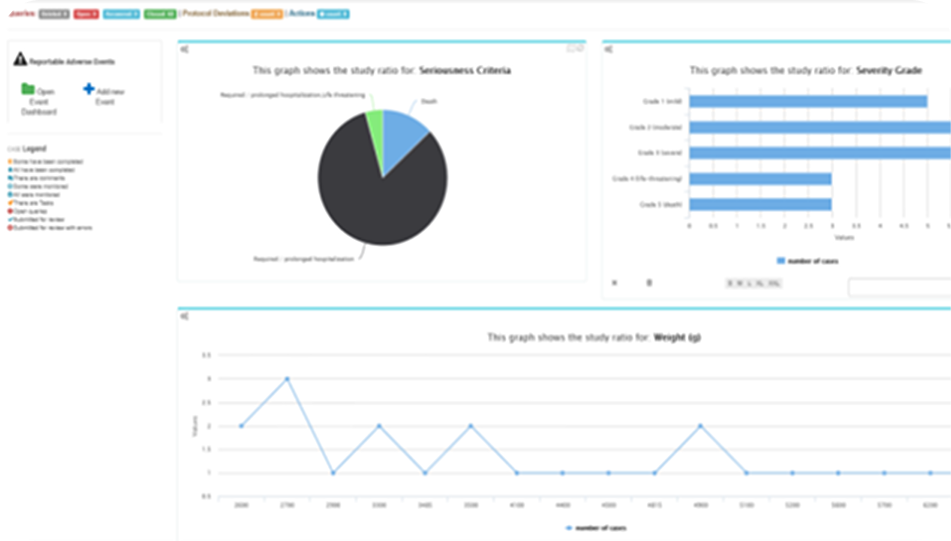
Dashboarding & Visualization:
Stay on top of your data with real-time dashboards and customizable visualizations. Our intuitive dashboard allows you to quickly view key metrics, track trends, and gain insights into the safety and quality profile of your products. With easy-to-read charts, graphs, and summaries, you can make informed decisions faster and manage risks more effectively.
Customizable Alerts and Notifications:
Stay on top of urgent safety and quality issues with customizable alerts and notifications that can be configured for specific events, users, or regions. Ensure timely follow-ups and investigations without missing critical information.
Data Privacy & Security:
Protect sensitive patient and product data with our robust security measures, including encrypted communication channels and role-based access controls. Rest assured that your data is safe and compliant with data protection regulations such as GDPR and HIPAA.
Audit Trails & Reporting:
Maintain complete transparency with detailed audit trails for every event reported. Generate comprehensive reports, summaries, and regulatory filings, reducing the time and effort needed.
Regulatory Compliance:
Ensure the highest standards of data integrity and security with comprehensive 21 CFR Part 11 and Annexure 11 compliance. Our system meets the FDA and EMA's requirements for electronic records and signatures, providing secure, traceable, and audit-ready documentation that supports both safety and regulatory audits.
Customizable Dashboards
The dashboards are a high-level overview of the combined system data. This is useful to drill into regional or functional groups to see the performance or statistical values thereof. The dashboard is configurable and permission driven.
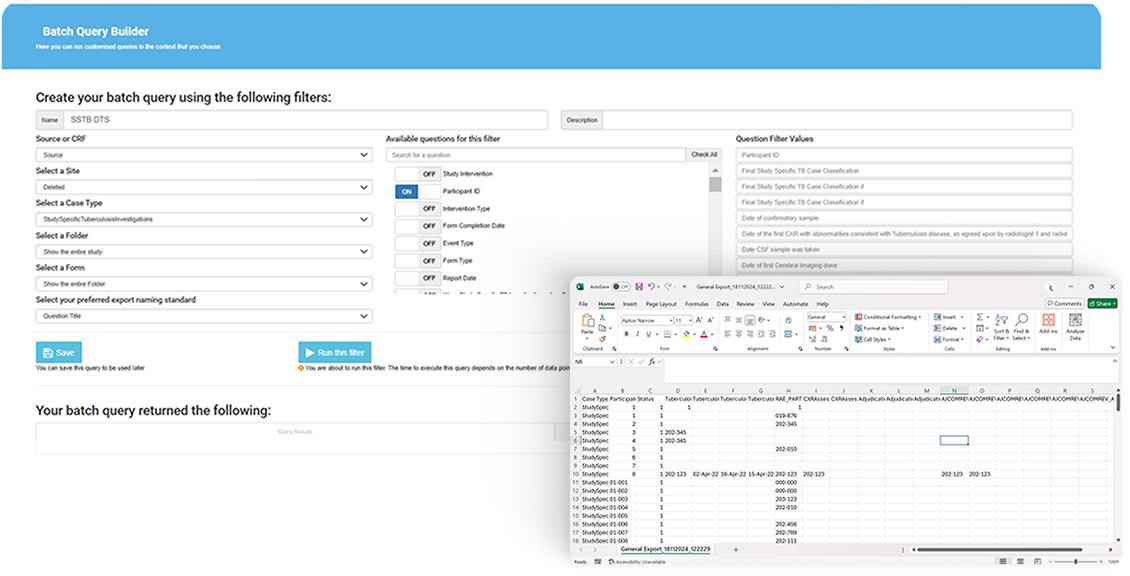
Reporting Wizard
The reporting wizard allows the user to dynamically create reports from different areas in the system using a number of predefined filters. These reports can be exported to PDF or CSV (using either SDTM, Normal or Custom formatting) to be customised further on other platforms.
Automated Letter Generation
Our automated letter generation tool is simple yet powerful. It allows you to automatically map information from the system into the configured spaces on the letters. This allows you to generate the letters accurately with the information captured in the system. The letters can be scheduled to send automatically based on events configured by the administrator or the letters can be manually configured and previewed by the system users.
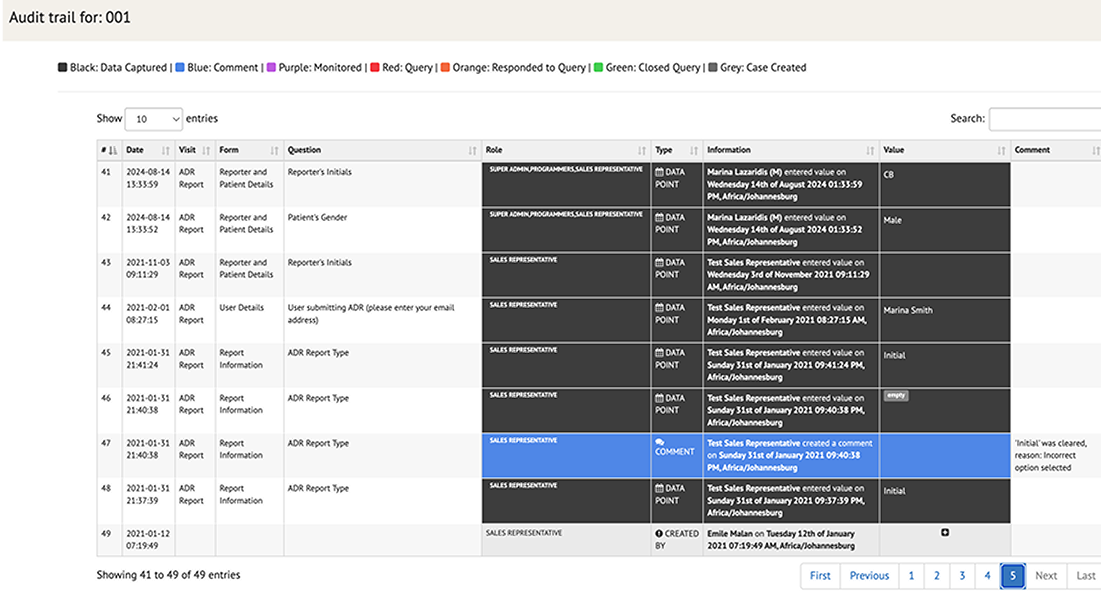
Audit Trails
Maintain complete transparency with detailed audit trails for every event reported. Generate comprehensive reports, summaries, and regulatory filings, reducing the time and effort needed for post-market surveillance.
Regulatory Compliance:
Our uniquely designed framework allows all the stakeholders within the product development lifecycle to work on a central, SOC-3 compliant cloud-hosted platform sharing the same database. The entire portal is governed using role-based access and has been implemented and built with the FDA’s CFR Part 11 regulations and HIPAA privacy laws as foundation.

Coming Soon
SafetyBase v3.0 Enhancements
ICH E2B R3 Compliance
Ensure global regulatory compliance with the latest E2B R3 standards for Individual Case Safety Reports (ICSRs). Our system supports the structured transmission of safety data, making it easier to submit accurate and compliant reports to health authorities worldwide.
Auto-Coding with MedDRA and WHO-DD Support
Streamline the reporting process with auto-coding capabilities. The system automatically maps event descriptions to standardized medical terminologies (such as MedDRA or WHO-DD), improving the accuracy and consistency of data entry while reducing manual effort and errors.
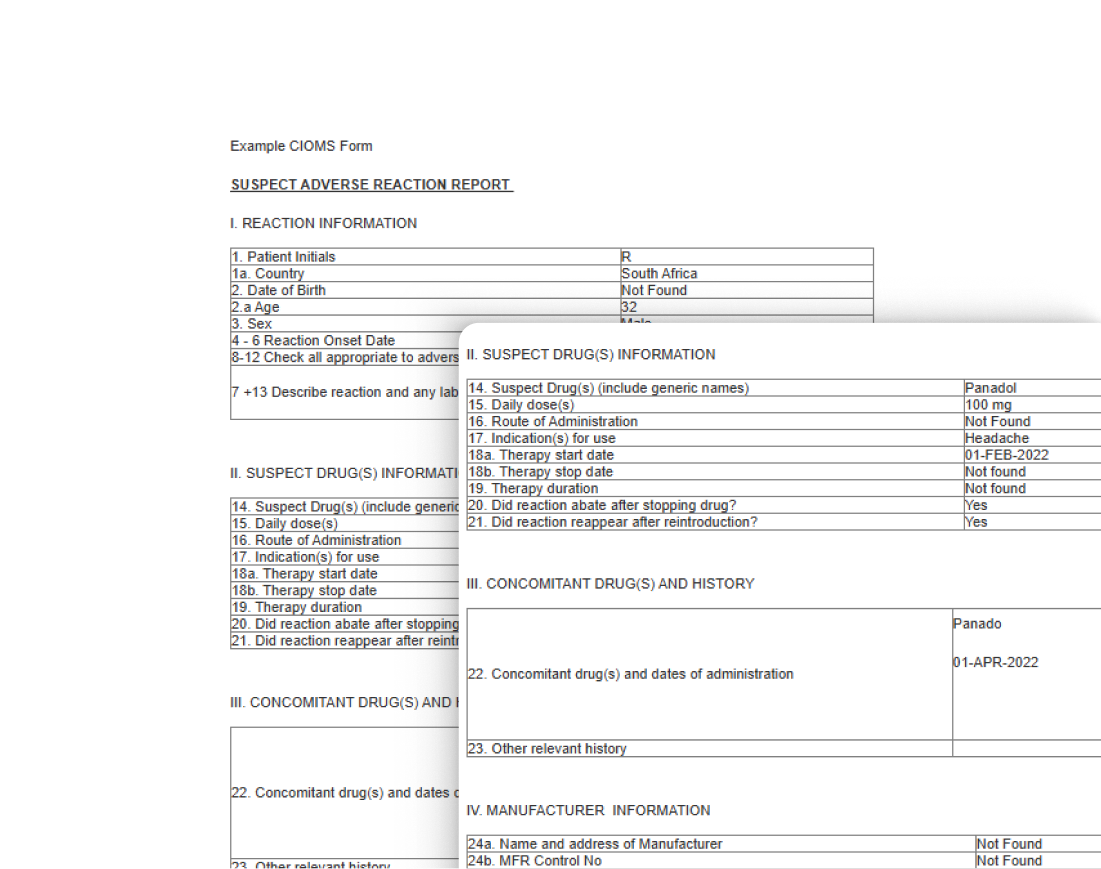
CIOMS Reporting
Effortlessly generate CIOMS (Council for International Organizations of Medical Sciences) compliant reports, ensuring you meet the reporting requirements for international regulatory agencies. Our system simplifies the creation of these complex reports, making global reporting easier and more efficient.
Customised Listings
Tailor your surveillance reports to your specific needs with customized listings. Generate reports based on specific products, regions, or categories, allowing you to monitor safety and quality data in the way that makes the most sense for your organization.
Signal Detection
Proactively identify potential safety risks with signal detection capabilities. The system uses advanced analytics to detect emerging safety signals by analyzing trends and patterns in your safety and quality data, helping you to quickly identify issues before they escalate.
Auto-Submission to Regulators
Automate the submission of reports to regulatory authorities. Our system integrates seamlessly with global regulatory submission platforms such as VigiBase, ensuring timely, accurate, and compliant submissions with minimal manual intervention.